Research in childhood cancer at the Institut de Recerca Sant Joan de Déu cover of Science Translational Medicine journal
The treatment consists of injecting a genetically modified virus into the eye affected by the tumour. The virus seeks out, attacks and destroys cancer cells, and is for use in children whose tumours do not respond to conventional treatments. The work appears on today's cover of the prestigious journal Science Translational Medicine.
The research has experimentally reproduced tumours obtained from patients who had not been cured with the treatments currently available. Researchers at Sant Joan de Déu and the biotechnology company VCN Biosciences have shown that the VCN-01 oncolytic virus, developed by genetic modification of adenovirus type 5—a common virus that normally causes cold-like symptoms—is only capable of infecting and multiplying in tumours and not in healthy cells of the retina. The virus's selectivity for tumours is based on the abnormal functioning of the retinoblastoma gene (RB1) in the cells affected by the tumour, in which there is an increase in the free amount of a molecule called E2F-1.
The VCN-01 virus was genetically modified by VCN Biosciences so that its replication is initiated by the presence of free E2F-1 in the cells it infects. This property causes the virus to replicate selectively in retinoblastoma cells, whereas it does not replicate in healthy retinal cells, where E2F-1 is not free but bound to the product of the RB1 gene.
SJD Barcelona Children's Hospital has begun a clinical trial, led by Dr Guillermo Chantada, Dr Jaume Catalá and Dr Jaume Mora, to treat patients with chemo-resistant eye tumours with the VCN-01 oncolytic virus. The objective of this experimental study, in which VCN Biosciences is also participating, is to describe the safety of the treatment and obtain the first indications of its clinical efficacy.
"Four years of work in the laboratory have been transformed into a clinical trial thanks to the committed support of the institutions and the involvement of professionals from multiple disciplines and hospital donors," explains Dr Ángel Montero Carcaboso, senior author of the publication and researcher in Institut de Recerca Sant Joan de Déu.
Dr Manel Cascalló, Executive Director of VCN Biosciences and an author of the work, stated that "The published data represent an important confirmation of the mechanism of action of our product, VCN-01, which is currently also being tested in adult patients suffering from other tumours, such as pancreatic cancer."
The work has been paid for in part through the Spanish Ministry of the Economy's competitive "Retos" [Challenges] and "Miguel Servet" programs. Dr Guillem Pascual, first author of the work, received the prestigious Schweisguth Prize from the International Society of Paediatric Oncology (SIOP) for this research. Other authors of the work belong to the Catalan Institute of Oncology (Barcelona), VCN Biosciences (Barcelona) and the Curie Institute (Paris), among other institutions.
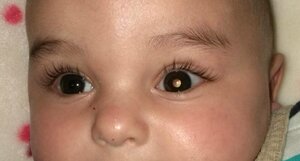
Retinal cancer is diagnosed in 8,000 children around the world each year. It is the most common ocular cancer in children. Currently, when ocular preservation is indicated, children receive intra-arterial chemotherapy in a first phase, which is administered through a long, thin catheter introduced through the femoral artery (in the groin) and running to the ophthalmic artery, where it then administers the chemotherapy locally.
Sometimes, chemotherapy drugs are injected directly into the eye, into the vitreous humour. In 30% of cases, however, the tumour does not respond to either of these treatments and ophthalmologists have no other option than to remove the affected eye to prevent the cancer from spreading to other organs in the body, since if this occurs, the likelihood of a cure is very low. The new viral treatment aims to prevent the removal of the eye and reduce cases of blindness in patients with retinoblastoma.
More information